There are many great challenges facing the cannabis industry. Because production and research have been limited for so long it has been difficult to explain the relationship between dried marijuana flower and yield of concentrate. As rules and regulations have been defined differently amongst multiple states it has become even more challenging to agree on what, if any, usable marijuana or concentrate weight limits should be applied. In this article we will look at how a few states are addressing the issue.
Colorado
Always a pioneer in cannabis regulation, in August of 2015, Colorado cannabis regulators published the “Marijuana Equivalency in Portion and Dosage” study. This study investigates marijuana equivalencies from three perspectives: production, price, and dosing.
Physical equivalencies were calculated in two ways; a THC equivalency, and a physical production equivalency. Equivalencies were calculated using the popular concentrate and infused product manufacturing techniques, including butane hash oil, CO2 oil, ethanol, and water. Physical production equivalency is calculated by isolating the biomass inputs and determining a yield ratio of usable marijuana. Edibles, refers to the yield of usable marijuana used for edibles and is reported in milligrams. The THC methodology provides an equivalent amount of oil required to produce products with a specific amount of THC. The calculations were created using products based on recent state testing information. Table ES-1 shows equivalency factors for both methodologies by solvent type.
The physical equivalencies in Table ES-1 show that
between 347 and 413 edibles of 10mg strength can be
produced from an ounce of marijuana, depending on the
solvent type and production method. For concentrates,
between 3.10 and 5.50 grams, with average potency, of concentrate are equivalent to an ounce of flower marijuana.
The conversion factors described above are the first of
their kind. They can be useful for understanding cannabis production
management processes.
The study published by CO also explores the different effects of inhaling concentrate compared to digesting concentrate. The study suggests limits based on a Pharmacokinetic understanding as well.
An important compliment to the physical THC relationships identified in this study, the pharmacological perspective. If the purpose of the equivalency legislation is to limit transactions or possession to a reasonable “dose” of concentrates and marijuana products then we must consider the medical effects..
California
Another common mistake the states have made is trying to set limits based on THC but not defining THC or not defining it properly. California defines “THC” as delta 9-tetrahydrocannabinol.
Cannabinoid content for cannabis and cannabis products in California must be included on either the primary or informational panel. For cannabis flower, cannabinoid content must be listed as a percentage. For cannabis products, cannabinoid content must be listed in milligrams and include delta-9 THC and CBD, at a minimum. However, the state does not note the difference between delta-9 THC and THCA (non activated THC). This could lead producers to oversell products or not report products that contain potentially psychoactive ingredients, although not activated unless heated.
For an example, §40315 regarding THC Concentration Limits says:
(a) An edible cannabis product shall not contain more than:
(1) 10 milligrams THC per serving; and
(2) 100 milligrams THC per package.
(b) Notwithstanding subsection (a), a package containing an edible product that is an orally-dissolving product, such as sublingual lozenges or mouth strips, may contain up to 500 milligrams THC per package, if:
(1) The cannabis product consists of discrete servings of no more than 10 milligrams THC per piece;
(2) The cannabis product is labeled “FOR MEDICAL USE ONLY;” and
(3) The cannabis product is only available for sale to a medicinal-use customer.
(c) A topical cannabis product or a cannabis concentrate shall not contain more than 1,000 milligrams THC per package.
(d) Notwithstanding subsection
(c), a topical cannabis product or a cannabis concentrate may contain more than 1,000 milligrams THC per package, but not more than 2,000 milligrams THC per package, if the product is labeled “FOR MEDICAL USE ONLY” and is only available for sale to a medicinal-use customer.
What if a producer used oil that was not decarboxylated, but could still be edible (or smokable)? Would these limits still apply? Does THC mean only delta-9 THC or the combination of both? The industry has established a formula to calculate Max THC, although state regulators have yet to adopt the use of this formula. This would ensure if a product contains cannabis oils, that has not been decarboxylated, it is understood that if heated, could have psychoactive effects.

While CA offers limits for packaging, there are also limit to what a cannabis retailer can sell and what a consumer can possess in CA. An adult-use cannabis customer in CA may purchase the following in a single day from a licensed retailer:
- Up to 28.5 grams of non-concentrated cannabis.
- Up to 8 grams of concentrated cannabis, including concentrate contained in cannabis products.
- Up to 6 immature cannabis plants.
Here, are we talking about 8 grams of oil or 8 grams of THC?
A medicinal cannabis customer with a physician’s recommendation may purchase the following in a single day from a licensed retailer:
- Up to 8 ounces of medicinal cannabis in the form of dried mature flowers or the plant conversion.
- Up to 12 immature cannabis plants.
- An amount of medicinal cannabis consistent with the patient’s needs as recommended by a physician.
Stay with us…
Arizona
Arizona has been examining this topic closely. While the medical program developed multiple court cases formed around the legality of cannabis concentrates. Arizona medical marijuana statutes define medical marijuana as:
“Marijuana”:
(a) means:
(i) All parts of any plant of the genus cannabis
, from which the resin has not been extracted, whether growing or not,and the seeds ofsuchTHE plant.Marijuana does not include the mature stalks of such plant or the sterilized seed of such plant which is incapable of germination.(ii) THE RESIN EXTRACTED FROM ANY PART OF A PLANT OF THE GENUS CANNABIS, AND EVERY COMPOUND, MANUFACTURE, SALT, DERIVATIVE, MIXTURE OR PREPARATION OF THE PLANT OR ITS SEEDS.
(iii) EVERY COMPOUND, MANUFACTURE, SALT, DERIVATIVE, MIXTURE OR PREPARATION OF THE RESIN OR TETRAHYDROCANNABINOL.
(b) INCLUDES PLANT MATERIAL FROM WHICH THE RESIN HAS NOT BEEN EXTRACTED.
(c) DOES NOT INCLUDE THE MATURE STALKS OF THE PLANT OR THE STERILIZED SEED OF THE PLANT THAT IS INCAPABLE OF GERMINATION.
And while most recently efforts have been made on the best way to regulate, not prohibit, concentrates, even the most recent changes to regulations have not addressed this complex issue .
Like most states AZ marijuana dispensary’s have both labeling requirements and sale limits. However, they have been even more vague in how much concentrate or marijuana edibles one can legally purchase. Arizona requires:
A. A dispensary shall ensure that medical marijuana provided by the dispensary to a qualifying patient or a designated caregiver is labeled with:
2. The amount, strain, and batch number of medical marijuana;
Arizona defines purchasing limits as two and one-half ounces of medical marijuana during any 14-calendar-day period. It is unclear how many edibles or grams of concentrate one can posses or purchase in AZ.
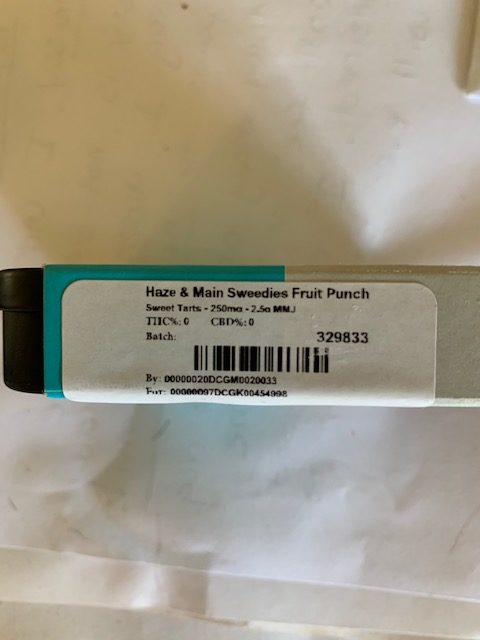
AZ does not currently require testing of product, although we expect that to change in the near future. While it is not uncommon to find THC and CBD content on product labels in Arizona, it is not technically required. The product such as an edible may list 250mg THC and 2.5 grams usable marijuana. 250mg does not = 2.5 grams. So, what is that the weight of? Most likely it is the weight of the oil or THC multiplied by the yield % (or an average yield %) of the original biomass source.
The state, the industry, the patients and the news have participated in many discussions regarding dry weight equivalents, however the topic remains unresolved. Product labels and amounts applied to limits appears inconsistent.
Oklahoma
The State of Oklahoma released emergency regulations in August 2018 to clarify key vocabulary. Interestingly their definition for concentrates specifies cannabinoids, although like most states, does not limit that to THC
“Marijuana” means all parts of a plant of the genus cannabis,
whether growing or not; the seeds of a plant of that type; the resin
extracted from a part of a plant of that type; and every compound,
manufacture, salt, derivative, mixture, or preparation of a plant of
that type or of its seeds or resin. “Marijuana” does not include the
mature stalks of the plant or fiber produced from the stalks; oil or
cake made from the seeds of the plant; or any other compound,
manufacture, salt, derivative, mixture, or preparation of the mature
stalks, except the resin extracted from the mature stalks, fiber, oil
or cake; the sterilized seed of the plant that is incapable of
germination; or industrial hemp, from the plant Cannabis sativa L. and
any part of such plant, whether growing or not, with a delta-9
tetrahydrocannabinol concentration of not more than three-tenths of
one percent (0.3%) on a dry weight basis the same as the term that is
defined in 63 O.S. § 2-101 .“Medical marijuana concentrate” (“Concentrate”) means a substance
obtained by separating cannabinoids from any part of the marijuana
plant by physical or chemical means, so as to deliver a product with a
cannabinoid concentration greater than the raw plant material from
which it is derived. Categories of concentrate include water-based
medical marijuana concentrate, food-based medical marijuana
concentrate, solvent-based concentrate, and heat- or pressure-based
medical marijuana concentrate as those terms are defined in the
Oklahoma Medical Marijuana and Patient Protection Act, 63 O.S. § 427.1
et seq.
“Medical marijuana product” means a product that contains
cannabinoids that have been extracted from plant material or the resin
therefrom by physical or chemical means and is intended for
administration to a qualified licensed patient, including but not
limited to concentrates, oils, tinctures, edibles, pills, topical
forms, gels, creams, and other derivative forms, except that this term
does not include live plant forms.
“Medical marijuana waste” means
Both possession and purchase limits defined by OMMA.
A patient who has been issued and is in possession of an OMMA
medical marijuana license is legally authorized to:
(1) Consume marijuana legally;
(2) Legally possess up to three (3) ounces (84.9 grams) of
marijuana on their person;
(3) Legally possess six mature marijuana plants;
(4) Legally possess six seedling plants;
(5) Legally possess (1) ounce (28.3 grams) of concentrated
marijuana;
(6) Legally possess seventy-two (72) ounces (2,037.6 grams)
of edible marijuana; and
(7) Legally possess up to eight (8) ounces (226.4 grams)of
marijuana in their residence.
These possession limits are cumulative and a licensed patient or
caregiver may possess at one time the totality of the items listed in
this Section.
Transaction limitations are identical, but do specify if a patient is under the age of 18 they are limited to once ounce of concentrate. It is unclear if 1 ounce or 72 ounces refers to the weights of the final product or if there are limits associated specifically with the weight of the cannabinoids. Assumably 72 ounces refers to the net weight of the product right?
Labeling requirements are simple reading:
Medical marijuana and medical marijuana
product labels shall contain, at a minimum, the following information:
(1) The Oklahoma Uniform Symbol in the manner and form prescribed
by the Department;
(2) THC potency;
(3) Terpenoid potency; and
(4) The statement, “This product has been tested for
contaminants.”
Labels for edible medical marijuana products shall also meet the
requirements set forth in OAC 310:681-5-8.1.
Which reads:
… See OK regulations for additional warnings and requirements.
(A) Name and address of the business;
(B) Name of the food;
(C) Net quantity or weight of contents;
(D) Ingredients list;
(E) Food allergen information; and
(F) Nutrition labeling, if required under 21 CFR § 101.9.
(3) In addition, principal display panels or information panels
must contain:
(A) List of cannabis ingredients;
(B) The batch of marijuana;
(C) The strain of marijuana (optional);
(D) THC dosage in milligrams per unit; and
(E) The lot code.
(4) Nutrient content, health, qualified health and
structure/function claims must comply with the Food and Drug
Administration (“FDA”) Food Labeling Guide.
Massachusetts
Massachusetts is also a particularly confusing state as they have separate methods for “medical” and “adult use” equivalency.
Adilas420 offer an online Massachusetts Responsible Vendor Course that explains in detail the differences between adult use and medical marijuana usable marijuana weights and dry weight equivalencies. MA new proposed regulations, discussed in this course, became more complex by defining new packaging and sales limits for THC.

Advice to the Goverments
If asked for my advice, I would suggest the state decides if limits will be based on the equivalency of usable marijuana weight (including terpenes and other plant material) AND/OR THC, but should keep them as separate equivalencies. A clear deffiniton of THC to include “active delta 9 THC” or non activated THCA in the regulations will help ensure consumer understanding and safety. If asked if any limit should be applied, I would pose the question: What are the goals of the limitations? Is the goal to try to prevent diversion, IE, if a dispensary were to issue too much people may give it away or sell it? Or is the goal to control how much psychoactive ingredients someone can purchase at once?
Also, knowing under the adult use model, one person could go to multiple dispensaries and purchase more and more product, and no customer record is required, what do these limits accomplish?
I am not suggesting no limits. I support limits of MAX THC content in a single edible or concentrate packaging. This helps to improve the consumer experience allowing them to better self control dosing and ensure consumer safety. While I can also see concerns with excessive packaging requirements having a high cost to businesses and the environment, we can all understand wanting to take safety precautions for consumers.
If the goal is to prevent black market sales, the best way to do that is to allow businesses to keep their prices low to compete with the black market operations. Allowing customers to purchase as much as they want, while paying tax, will regulate supply and demand. Seed to sale tracking, proper labeling and consumer education will help ensure consumer safety.
Let Adilas420 help! Get help deciphering the rules for your state from an experienced cannabis consultant. Contact us today to get started!